Research Overview
The Nag lab develops novel peptide or peptidomimetic macrocycle libraries though optimization of robust chemical reactions such as Copper catalyzed Alkyne-Azide Cycloaddition (CuAAC) reactions for solid phase synthesis. We utilize different properties of these novel macrocycles, such as their modular natures and possible facile modifications of chemical properties for achieving our research objectives. Our research also focuses on developing new screening techniques and on understanding cellular phenomenon such as cellular uptake of peptides at a molecular level. Another aspect of our research is on developing new principles and techniques of engineering peptides, to engineer desired properties such as high protease resistance and high selectivities for their targets in complex environments.
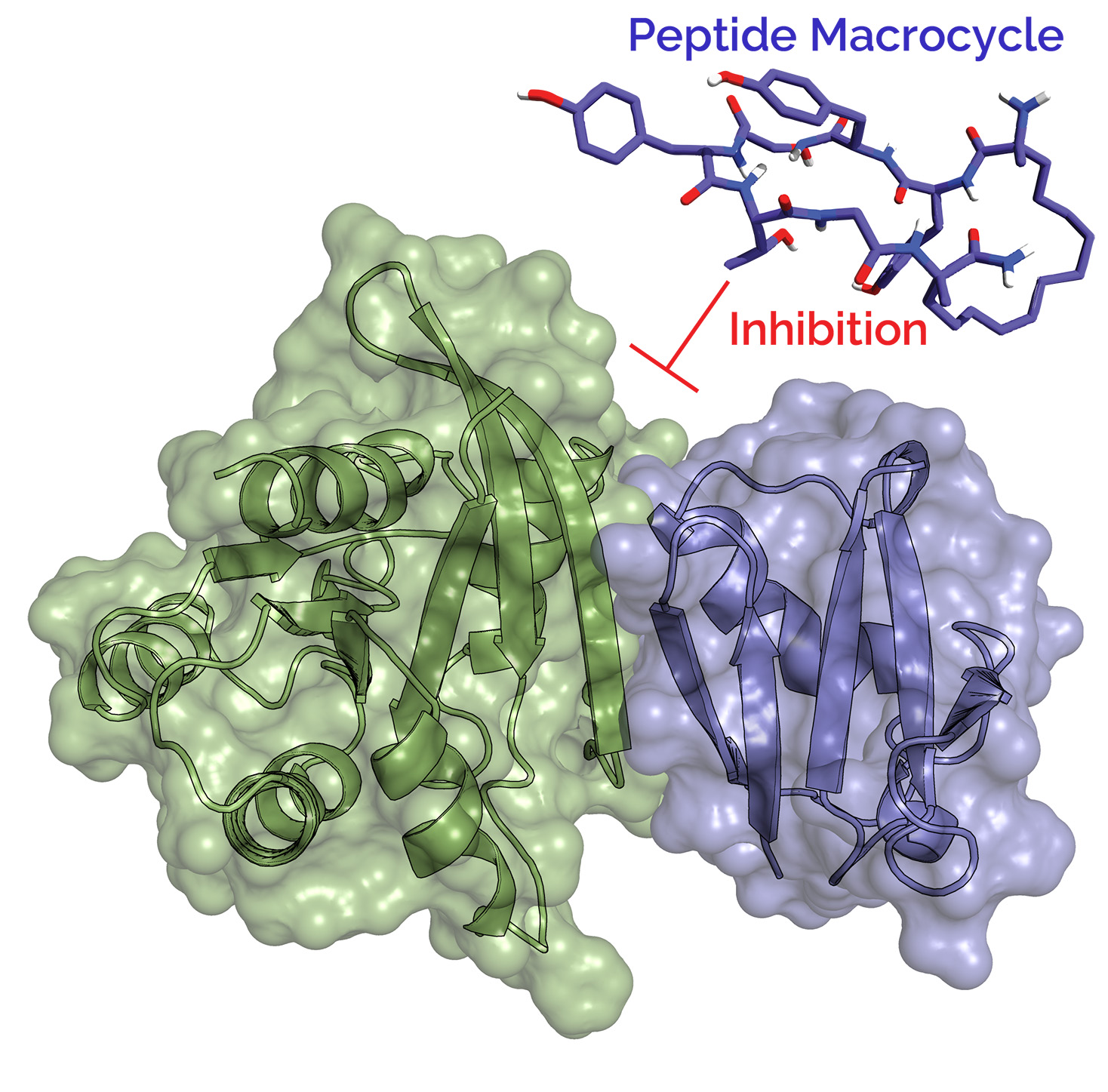
Macrocyclic peptides as potential Cancer Therapeutics
Peptides, and particularly macrocyclic peptides have gained renewed interest in the last decade as drugs, with FDA approval of around 30 macrocyclic peptide drugs. Macrocyclic peptides bridge the gap between small molecule drugs and protein based drugs such as therapeutic humanized antibodies – they have intermediate sizes of approximately 1000, have high protease stability, and various functionalities can be easily incorporated in macrocycles.
Intracellular regulatory proteins involved in cell signaling frequently interact with each other through large surface areas, a phenomenon termed as Protein Protein interactions (PPi). PPis lead to aberrant cell signaling events that cause cancer, and are notoriously difficult to inhibit by small molecule drugs. Peptide based macrocycles are ideal candidates to prevent intracellular PPi, as their extended structures can conform to the large protein surface areas involved in PPi. The Nag lab is developing a technology to synthesize and screen novel peptide macrocycles which can penetrate the cell membranes and selective target the protein interaction surfaces. These macrocycles will therefore act as potential therapeutic leads for cancer treatment.
Developing biological small molecule sensors
Building on our previous research on selective recognition of phosphorylated serine and phosphorylated proteins, the Nag laboratory plans to develop rigid macrocyclic reagents for recognition of phosphate containing biological small molecules. Selective recognition will involve not only the phosphate moiety sensing but also unique sensing of other parts of the small molecule. Rigid scaffolds will be utilized for phosphate recognition while the variable amino acid/small molecule components of the macrocycle will allow selective recognition of the small molecule.
Designing biomimetic catalytic centres
A grand challenge for scientists in the twenty first century is developing efficient solar fuel cells for energy generation. As protein complexes, such as Photosystem 1 and Photosystem II are very efficient catalysts in the plant cellular environment, scientists initially pursued studies immobilizing these proteins on electrodes for photosynthetic processes of water oxidation and carbon dioxide reduction. However, these proteins degrade rapidly outside the cellular environment. On the other hand, metal clusters that act as the enzymatic centers in plant cells cannot perform catalysis without the cellular protein environment. In the Nag Lab, we are developing a series of metal-ligand frameworks, where the ligands are peptidic macrocycles and explore their water oxidation and carbon dioxide reduction properties. Peptide macrocycles are in between proteins and enzymatic centers in size, but their restricted geometries can help provide molecular contacts for catalytic activities, while modifications will be made to stabilize these compounds more than proteins.